Imagine a future where surgical implants simply dissolve away once their job is done, eliminating the need for follow-up surgeries. This is the promise of biodegradable implants, but creating one that’s strong enough to support bone, biocompatible, and degrades at a controlled rate is a formidable challenge.
Magnesium alloys are emerging as strong contenders for these temporary implants. While magnesium offers excellent mechanical properties and biocompatibility, its rapid corrosion within the body has been a major stumbling block. Researchers worldwide are actively exploring methods to slow this degradation, paving the way for wider use of magnesium-based implants. The goal is to balance degradation with bone recovery. If it vanishes too soon, complications arise. If it lingers, the body must work hard to remove it.
One promising approach involves coating the magnesium alloy with a biodegradable polymer. This creates a physical barrier, slowing down the corrosion process. A team led by Vijayshankar Dandapani at the Indian Institute of Technology (IIT) Bombay recently investigated the effectiveness of a specific polymer coating, poly(3-hydroxybutyrate) (PHB), on a magnesium alloy called ZK60 (Mg-Zn-Zr). ZK60 on its own typically degrades within about 12 weeks.
Dandapani and his team published their findings in Electrochimica Acta. Their work focused on mimicking the body’s natural environment as closely as possible. “We thought it would be worthwhile to study the corrosion of a PHB-coated ZK60 and measure quantitatively the extent of degradation using an ensemble of characterization techniques,” the researchers stated.
“The novelty of the work lies in using an electrolyte flow system to study the corrosion of a polymer-coated magnesium alloy,” they wrote.
The team used a dynamic electrolyte flow system to simulate the fluid environment within the body. This is crucial, as static immersion tests don’t accurately reflect the constant flow of bodily fluids around an implant. This dynamic environment can significantly impact the corrosion rate.
Initial tests compared the corrosion of bare ZK60 alloy in both static and dynamic conditions. The Unexpected Anomaly came when they found the dynamic electrolyte flow drastically accelerated corrosion compared to static immersion. Immediate Reaction was that they needed to redesign the barrier coating for the implant. The Lingering Question: would a better barrier have positive results?
They discovered that the flowing electrolyte prevented the formation of protective salt precipitates on the alloy surface, leading to faster degradation. The scientists reasoned that the constant exchange of ions by the flowing electrolyte hindered the formation of these protective layers.
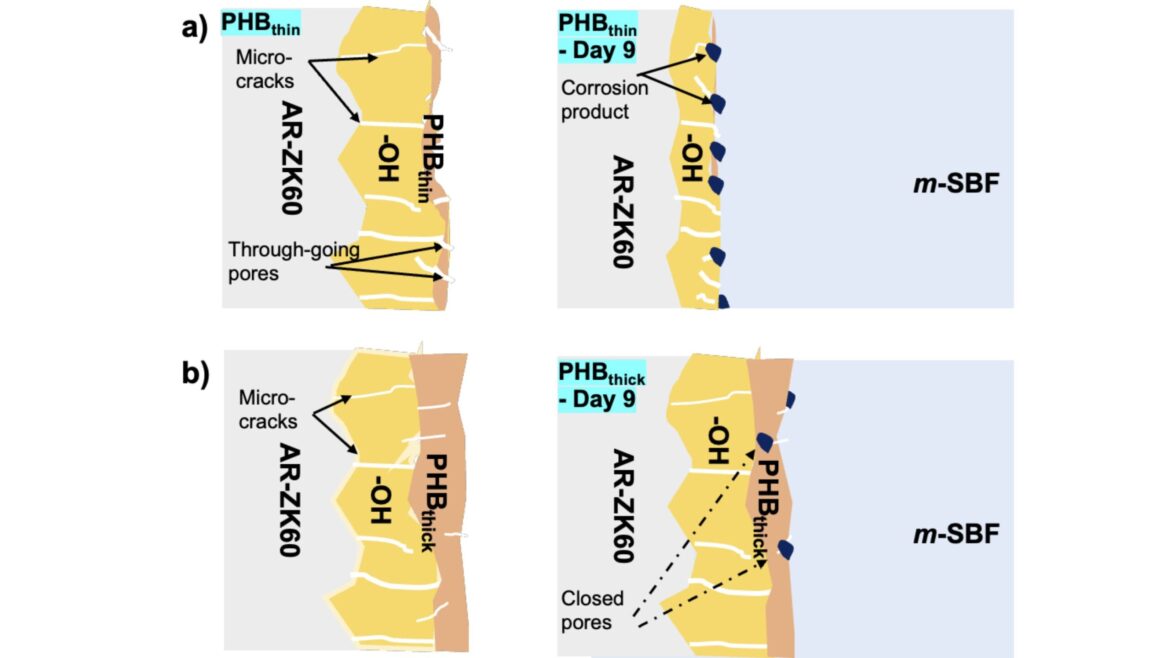
To combat this, the team pre-treated the ZK60 alloy with sodium hydroxide and then applied PHB coatings of varying thicknesses. They referred to these coatings as PHBthin and PHBthick.
Microscopic analysis revealed that PHBthin coatings contained numerous pores, while PHBthick coatings were relatively pore-free. The team then evaluated the ability of these coatings to retard corrosion under dynamic flow conditions over a nine-day period.
Here’s a summary of their key findings:
- PHBthin coatings degraded more rapidly due to electrolyte penetration through the pores.
- PHBthick coatings provided significantly better protection due to their lower pore density.
- The extent of degradation could be controlled by tuning the pore density of the polymer coating.
“Using EIS, we measured a much lower ‘polarization’ resistance after nine days for PHBthin showing that it was much more degraded than PHBthick. We attributed this to the ‘through-going’ pores which allowed easy ingress of electrolyte and corroded the alloy,” the researchers explained.
The findings suggest that the thickness and density of the polymer coating are critical factors in controlling the corrosion rate of magnesium alloys. By carefully tuning these properties, researchers can tailor the degradation rate of the implant to match the healing process of the bone.
A local surgeon, Dr. Anya Sharma, commented on the potential impact of this research. “The idea of a temporary implant that degrades naturally is incredibly appealing. It reduces the need for additional surgeries and potentially minimizes complications for the patient. But the devil’s always in the details. Controlling that degradation, making sure it happens at the right pace, is key.”
However, the study also raises further questions. “It remains to be seen whether the corrosion products formed within the pores of the polymer coating provide protection over extended time,” Dandapani’s team noted. This is a crucial area for further investigation, as the long-term behavior of these corrosion products could impact the implant’s overall performance. It wasn’t what anyone expected, but the thick coating’s success opened new paths.
Another important consideration is the inflammatory response to the polymer and its degradation products. While PHB is generally considered biocompatible, long-term studies are needed to fully assess its safety in this application. It’s all about controlling the controllables, even the un-controllables.
Despite these challenges, the research offers a promising step forward in the development of biodegradable magnesium implants. By carefully controlling the properties of the polymer coating, scientists can potentially create implants that provide temporary support to the bone while it heals, and then gradually dissolve away, leaving no trace behind. One typos can throw the whole project off course.
For now, the team is focused on refining the polymer coating techniques and conducting longer-term studies to evaluate the performance and safety of these coated magnesium alloys. These experiments may not have worked out they way they hoped.
Science X Dialog facilitates the communication of published research findings. Learn more about Science X Dialog.
More information: G. Keerthiga et al, Understanding the corrosion behavior of poly(3-hydroxybutyrate) coated ZK60 Mg alloy under dynamic m-SBF electrolyte flow conditions, Electrochimica Acta (2025). DOI: 10.1016/j.electacta.2025.146812
Vijayshankar Dandapani is an Associate Professor at IIT Bombay, specializing in electrochemistry and corrosion.
ARTICLE_END